Medicalgorithmics has initiated its involvement in providing cardiac safety services for a pioneering clinical trial of a new medication. The trial, now in the drug dosing phase, utilizes Medicalgorithmics’ advanced AI software and ECG diagnostic devices. This initiative is a part of Project OATD-01, carried out for the Polish company Molecure and managed by Simbec-Orion. This exemplifies a significant expansion for Medicalgorithmics into the domain of cardiac safety in clinical trials.
This move signifies a new direction for Medicalgorithmics, venturing into the provision of cardiac safety services in clinical trials. The project’s goal is to determine the safety and effectiveness of the oral CHIT1 inhibitor (OATD-01) in treating approximately 100 patients with active pulmonary sarcoidosis, involving collaboration with over 20 prestigious medical institutions across the USA, European Union, Norway, and the United Kingdom. French CRO Simbec Orion, responsible for the trial’s organization and execution, selected Medicalgorithmics for its ECG cardiac safety services. Through this project, Medicalgorithmics monitors the heart function of patients exposed to the test substance, ensuring the highest standard of cardiac safety.
“After nearly a year of preparations, the clinical trial we are contributing to has advanced to the critical phase of new drug testing. This marks a significant milestone for us, confirming the commercial viability of our artificial intelligence-based system for heart monitoring and diagnostics in providing cardiac safety services. Offering patients involved in such projects the highest level of protection against cardiac risks has become a standard in the new drug market introduction process. The demand for such services is rapidly increasing, and Medicalgorithmics stands out as one of the few providers equipped with state-of-the-art AI algorithms and technology to support clinical trial efforts in this domain,” explains Magdalena Ślusarczyk, Director of Clinical and Product Implementation at Medicalgorithmics.
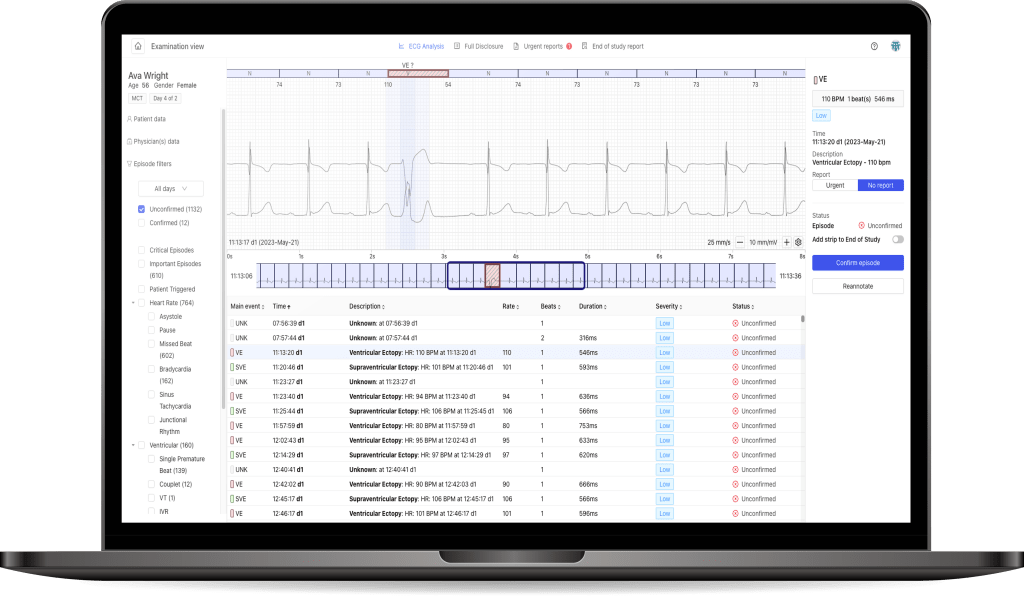
In line with its 2023-26 development strategy, Medicalgorithmics aims to expand its commercial involvement in clinical trials as a new business avenue. Currently, the company is engaged in two projects of this nature, with additional projects in the negotiation stage. Besides its collaboration with Simbec Orion, it also offers cardiac safety services for the GOAL-HF clinical trial, conducted by the Swedish biopharmaceutical company AnaCardio. Both projects utilize PocketECG devices for heart function monitoring, along with a diagnostic platform. Supported by AI algorithms, this system enables the real-time remote monitoring of patient heart function during new substance trials.
Medicalgorithmics is actively discussing participation in more international clinical research projects as a provider of cardiac safety services, expecting to finalize two agreements in this field within the first half of the year. One of these projects, involving up to 2,000 patients, will be carried out in the USA, Canada, and five European countries.
“The development of services for clinical trials represents a new revenue stream for our company, in addition to already acquired new partners, including four IDTFs from the United States this year. Thus, we are moving closer to achieving our goal announced during the recent Investor Day, which is to surpass the monthly revenue level of the second half of 2023 in the latter part of this year. Each new partner not only increases the profitability of our business but also enhances our ability to generate positive cash flows, which will positively impact the financial results of the company” – highlights Maciej Gamrot, CFO and board member responsible for finance at Medicalgorithmics.
About:
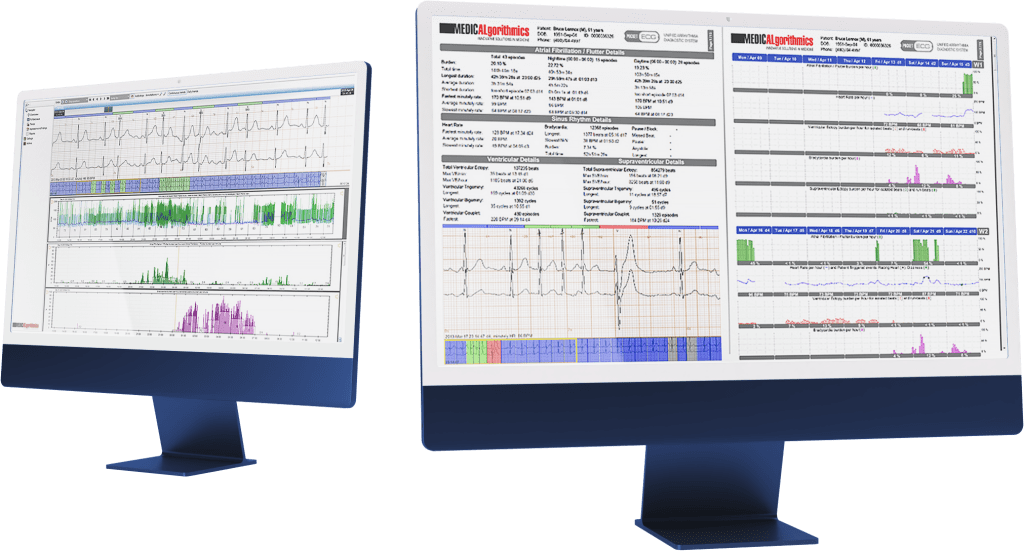
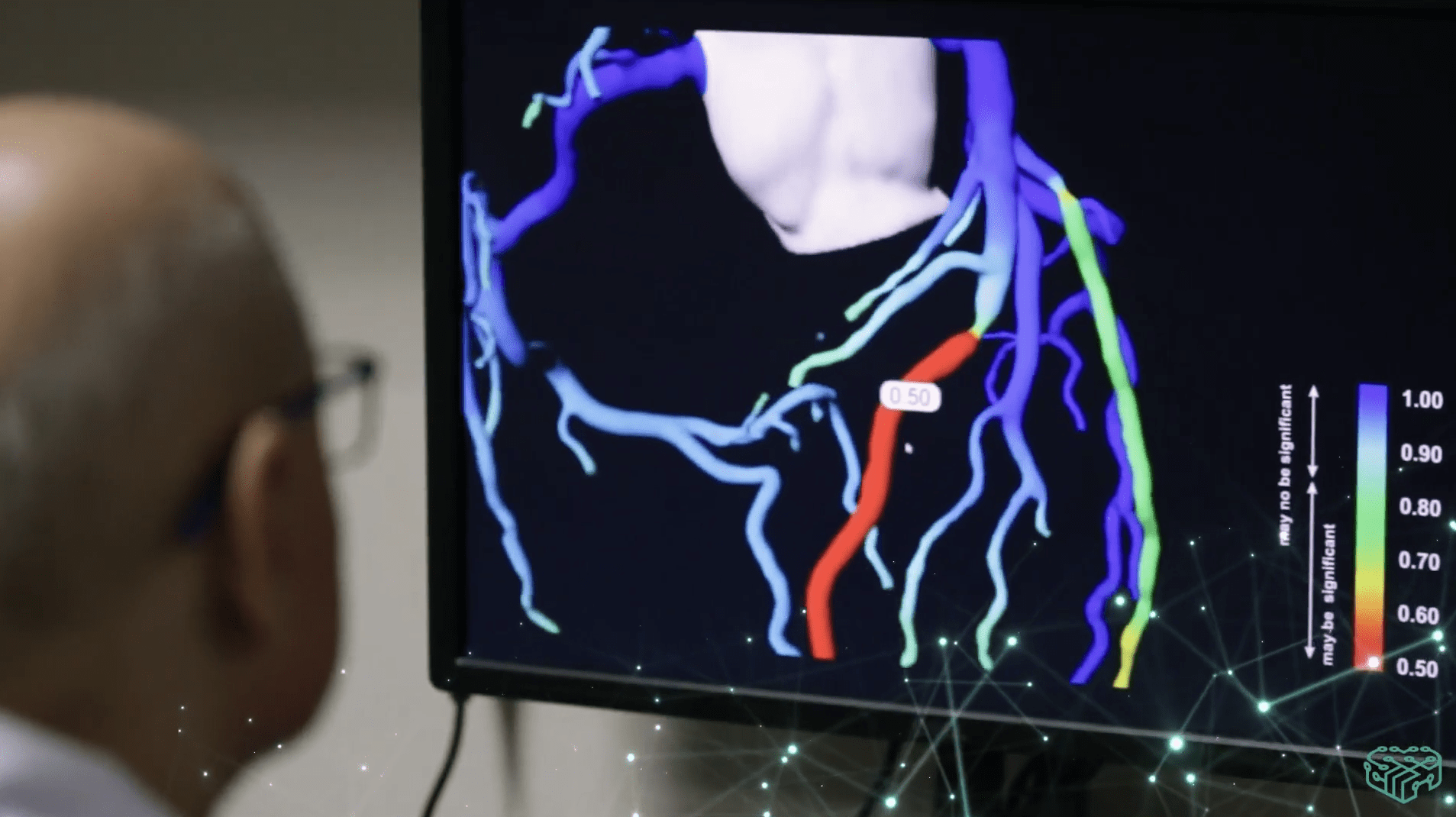
Medicalgorithmics is a global technology provider of non-invasive cardiac diagnostics monitoring software based on artificial intelligence. The company uses its proprietary AI system for ECG arrhythmia diagnostics. AI algorithms enable heart function monitoring analysis, cardiological analysis, and diagnosis. The Polish company’s solutions streamline work processes by optimizing the time and resources needed for cardiological study analysis. Its arrhythmia diagnostic software can detect 26 types of arrhythmias and cardiac abnormalities, one of the best results worldwide. The company also develops and commercializes VCAST technology, enabling non-invasive measurement of blood flow pressure in selected coronary vessels based on computer tomography images.
According to the development strategy announced in June 2023, created under the leadership of Medicalgorithmics’ main shareholder – Biofund Capital Management LLC – the company focuses on selling a platform for analyzing multi-day ECG records along with proprietary AI algorithms as a standalone product and integrating them with devices and IT systems of partners. Medicalgorithmics software can be licensed by clients, and the company will receive compensation in various models, including one based on the number of EKG data analyses performed.
* End *