Implementing its ongoing strategy, Medicalgorithmics, a medtech company listed on the sWIG80 index, has successfully
countries where we offer products and services
types of heart beats and arrhythmias detected
annotated heart beat library
We have a clear goal: improving health through intelligent technologies.
Medicalgorithmics Group was established in 2005 and is headquartered in Warsaw, Poland. Over the past two decades, we have transformed into a global provider of cutting-edge solutions that help improve the health of patients around the world. Today, the Group operates globally through four businesses to address today and tomorrow’s healthcare needs.
Medicalgorithmics has extensive experience in developing complex algorithms for the analysis of physiological signals, such as electrocardiograms (ECGs), as well as cardiac images.
DeepRhythmAI (DRAI) is a cutting-edge AI technology by Medicalgorithmics for heart rhythm analysis. It processes multichannel ECG data, delivering accurate results quickly, ideal for healthcare professionals managing abnormal heart rhythms.
The Virtual Cardiac Stress Test (VCAST) is a patented, AI-based medical system developed for non-invasive, clinical quantitative and qualitative analysis of CT-scan data, to assess the hemodynamic significance of coronary artery atherosclerotic stenosis.

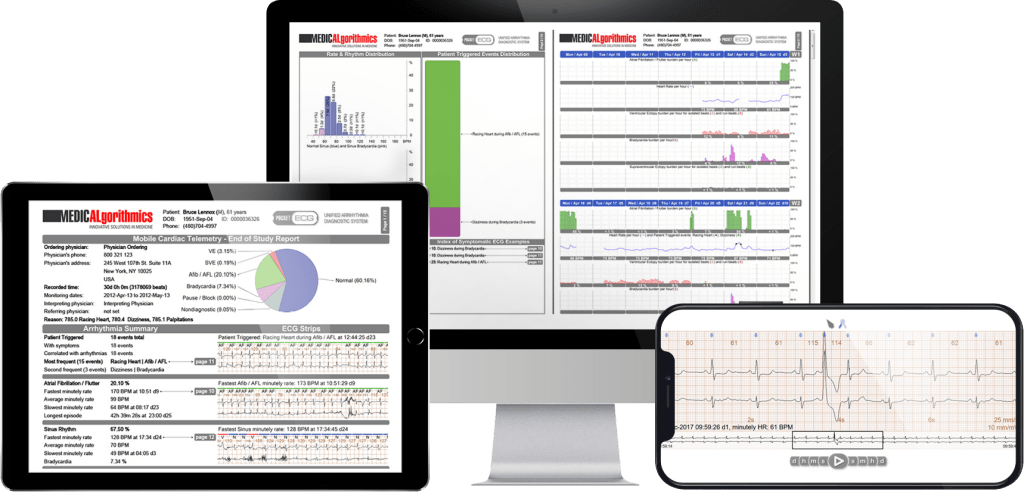
PocketECG is a complete diagnostic technology for detecting cardiac arrhythmias, which gives the doctor access to the full ECG signal throughout the test and the richest diagnostic report on the market with a full statistical analysis of the data.
About us
Our History
Management Team
Partners
Work with us
Job offers
Recommend an employee
Application form