Medicalgorithmics has received the CE certification allowing the PocketECG IV system and its artificial intelligence (AI)-powered software to be used in the European Union markets. The CE certification indicates that Medicalgorithmics’ PocketECG IV AI-based platform meets the quality and safety requirements of the Medical Device Regulation (MDR).
“The CE certification paves the way for us to sell PocketECG IV in the European Union and in markets outside the EU that recognize the CE mark. PocketECG IV was recently approved for use in Canada, which is our second most important market after the US. We are now working on obtaining approval to sell our platform in more markets,” says Jarosław Jerzakowski, Chief Commercial Officer at Medicalgorithmics. “PocketECG IV is compatible with LTE technology and meets the requirements for transmitting ECG data for years to come, increasing the attractiveness of our solution for medical facilities” – concludes Jarosław Jerzakowski.
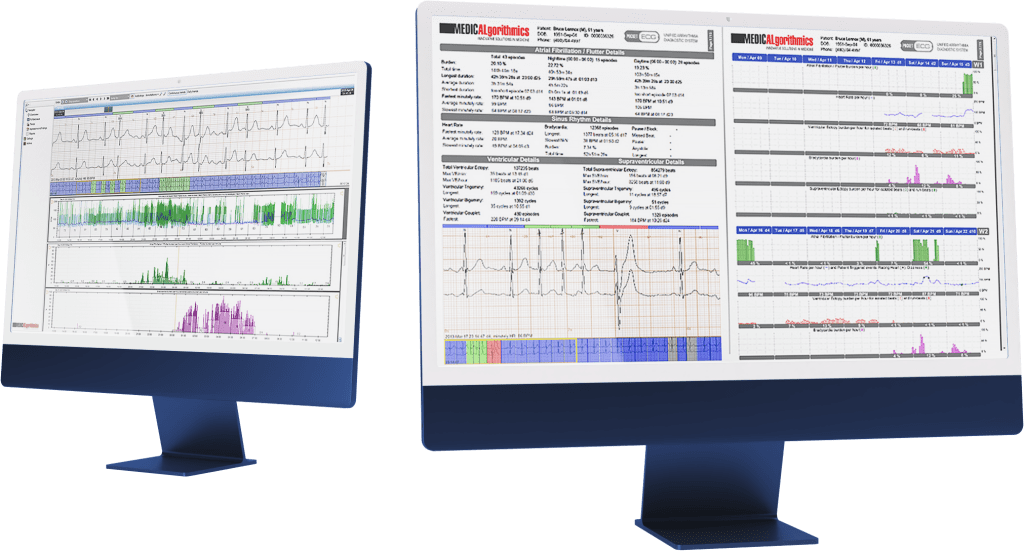
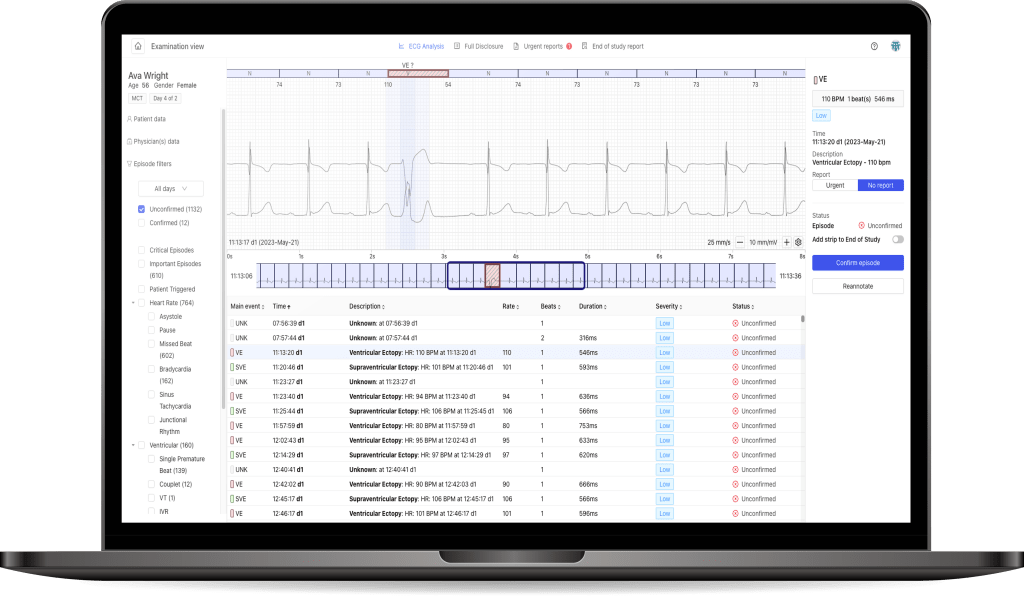
PocketECG IV is an advanced technological platform for arrhythmia diagnosis. The fourth generation allows for connecting the PocketECG device via LTE band, ensuring even more efficient transmission of continuous ECG signals.
“Since the first version of PocketECG, we have put emphasis on real-time transmission of the entire recorded ECG signal. By adding proprietary AI algorithms that analyze the recorded signal in real-time, we have created a unique system over the years that provides physicians with real-time information about the monitored patient’s condition. Our technology offers the highest quality analysis and gives physicians more leeway in deciding when to monitor the patient,” says Przemysław Tadla, Chief Technology Officer at Medicalgorithmics.