The rapidly increasing demand from the biopharmaceutical industry for services ensuring the cardiac safety of clinical drug trials represents a strong new trend in the global medical technology market. Medicalgorithmics, a medtech company listed on the sWIG80 index, intends to benefit from this trend by focusing on the development of high-margin services in this area. Last year, the company joined two international research projects and is now close to finalizing additional agreements and plans to collaborate with entities organizing clinical trials.
In June 2020, amidst the first wave of the COVID-19 pandemic, the U.S. Food and Drug Administration (FDA) unexpectedly revoked its three-month-old authorization of hydroxychloroquine for treating coronavirus infections. It was found that larger doses of this common malaria drug could cause heart arrhythmias and even cardiac arrest. This incident changed the regulatory approach to the approval process for new drugs. The FDA began requiring manufacturers to verify not only the effectiveness of new active substances but also their impact on heart function during clinical trials.
“The FDA’s new requirements have increased demand from biopharmaceutical companies for services related to heart function monitoring and diagnostics. In particular, the industry is looking for solutions that allow remote and near-real-time monitoring of patients participating in commercial clinical trials. Medicalgorithmics is capable of providing such services globally. Therefore, this year, in line with our strategy, we aim to grow dynamically as a provider of cardiac safety solutions in clinical research,” says Magdalena Ślusarczyk, Director of Clinical Affairs and Product Implementation at Medicalgorithmics.
As outlined in Medicalgorithmics’ strategy published in June 2023, commercial clinical trials are to become one of the new pillars of the company’s business. Last year, the medtech secured its first contracts in this area. In April, Swedish biopharmaceutical company AnaCardio selected it to provide cardiac safety services for the GOAL-HF1 clinical trial. In July, Medicalgorithmics signed an agreement with French firm Simbec-Orion, which is conducting a clinical research project on behalf of Polish company Molecure. Both projects are currently underway, and the company is monitoring cardiac safety in clinical trials at renowned American hospitals such as Mayo Clinic Rochester and Temple University.
“We are in advanced discussions to participate in more international clinical research projects as a provider of cardiac safety services. We may sign two agreements in this area in the first half of the year. One of the projects will be conducted in the USA, Canada, and five European countries and may involve up to 2,000 patients,” says Ślusarczyk.
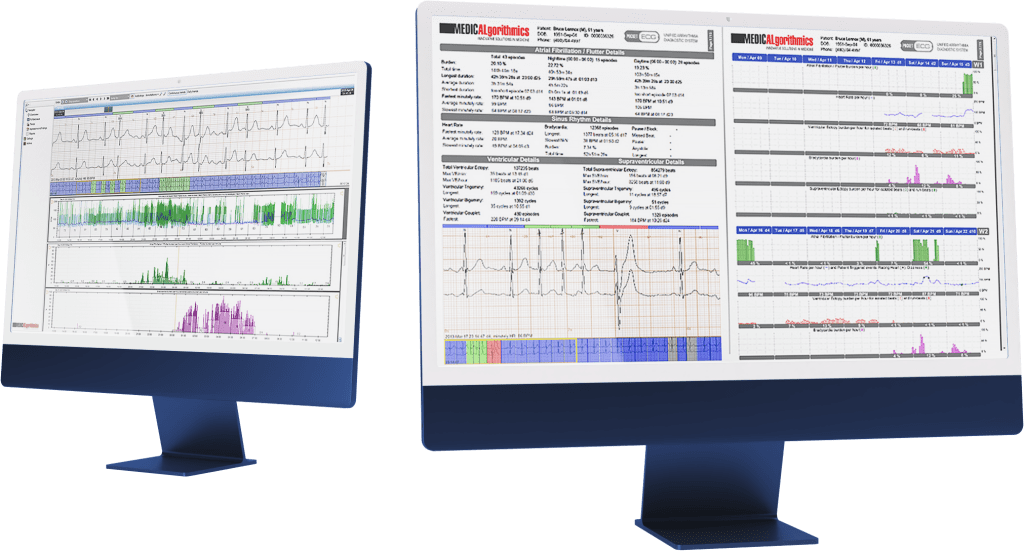
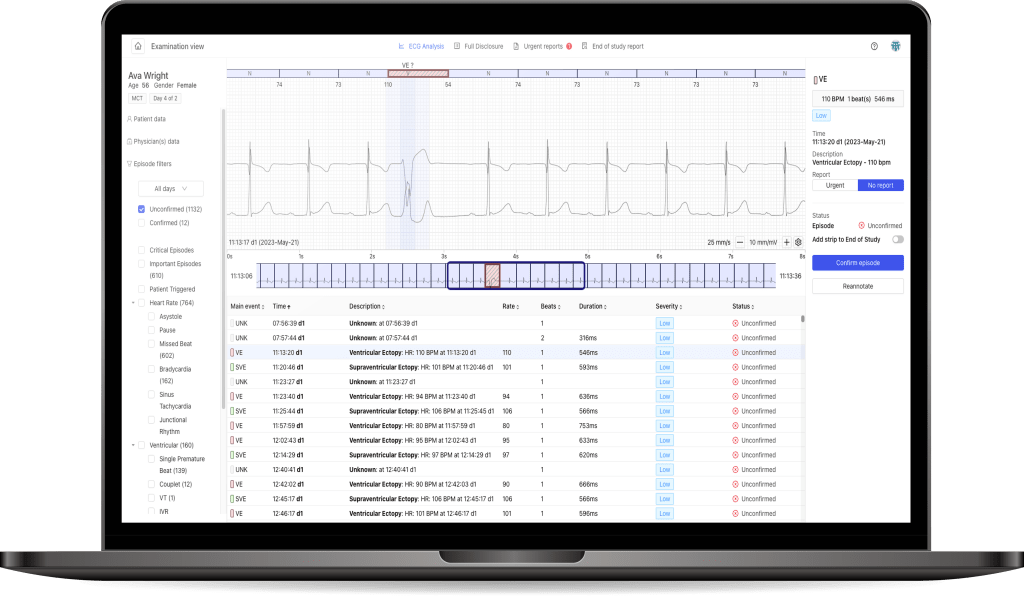
During clinical trials, clients can use the PCClient system with AI algorithms for heart monitoring. This includes the online PocketECG P4 device and a diagnostic platform that uses artificial intelligence algorithms to analyze collected data. This allows patients taking new drugs to be discharged to their homes instead of staying in the hospital. Additionally, Medicalgorithmics’ solution detects 26 types of arrhythmias and is certified in over 50 countries, enabling extensive cardiac support for clinical trial execution.
Medicalgorithmics expects that with the acquisition of additional projects, the scale of this activity and its recognition as a provider of cardiac services in the global clinical research market will increase. The company has mapped dozens of leading Contract Research Organizations (CROs), which organize clinical trials on behalf of biopharmaceutical companies, and intends to present them with its offer of cardiac safety services.
“Clinical trials represent not only a new revenue stream for Medicalgorithmics but also an opportunity to increase the business’s profitability. We perform cardiac safety services using the same resources that serve our patients in the area of arrhythmia diagnostics. However, due to the innovative nature of clinical trials, we can count on significantly higher rates than those for standard procedures covered by public or private healthcare funding systems. Hence, the development of clinical trials can have a significant positive impact on Medicalgorithmics’ revenues and profitability,” says Maciej Gamrot, CFO of Medicalgorithmics.
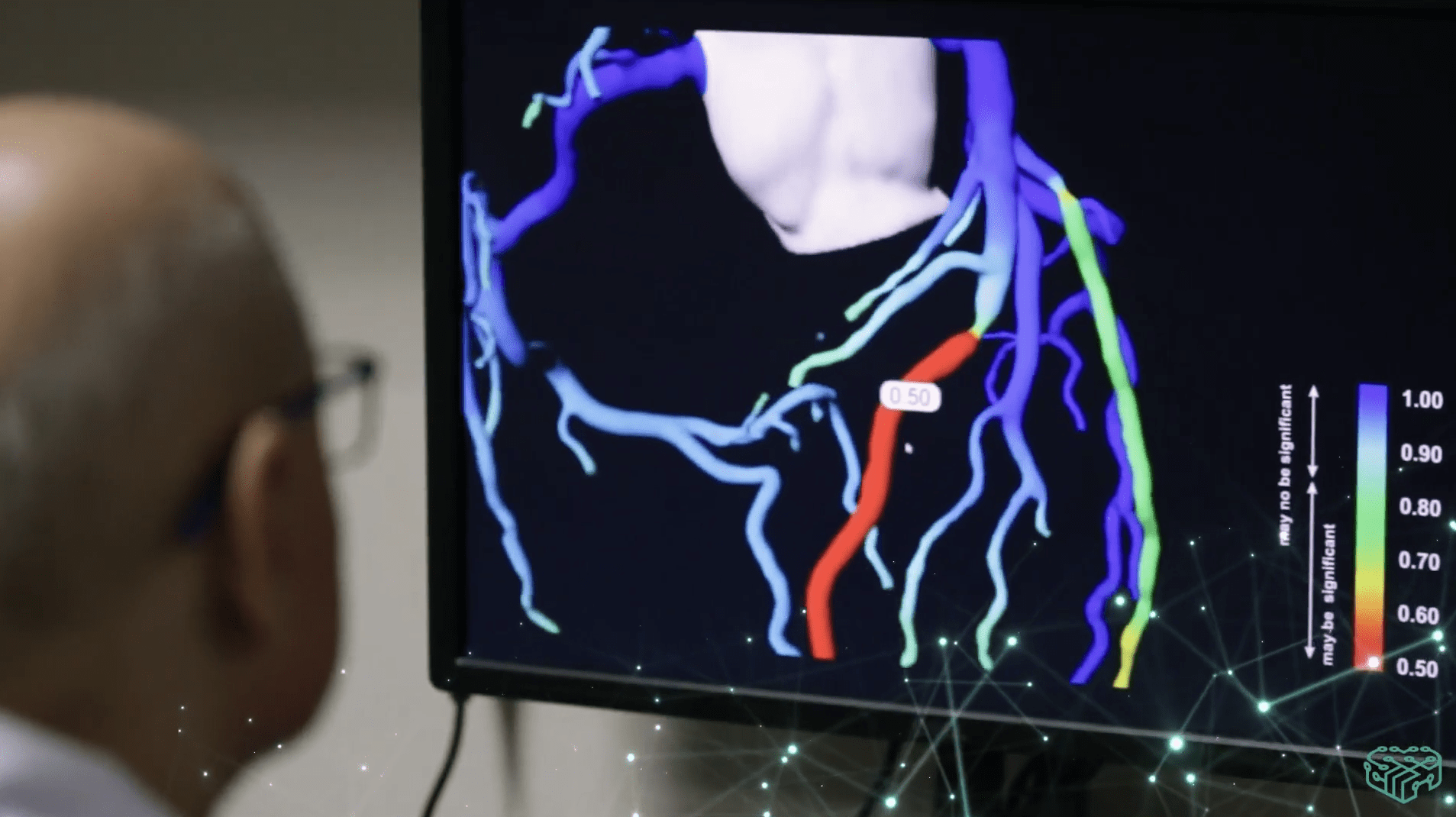
Following the development strategy announced in June 2023, created with the participation of Medicalgorithmics’ main shareholder, Biofund Capital Management LLC, the company has changed its business model. It moved away from offering only a closed AI cardiac diagnostics system tied to its own heart monitoring devices. In the new strategy, it focuses on selling an EKG signal analysis platform with its proprietary AI system as a standalone product and integrating it with the devices and IT systems of partners. Medicalgorithmics’ software can be licensed by clients, and the company will receive compensation in various models, including based on the number of EKG data analyses performed. Medtech is also developing VCAST technology, which will allow non-invasive diagnosis of coronary artery disease using artificial intelligence.
*End*
Investor Relations Contact:
Mateusz Paradowski
InnerValue
+48 516 089 279
Media Contact
Mariusz Gawrychowski
InnerValue
+48 501 520 598