Lightweight Holter monitor for 24 hours up to 18 days of continuous recording. The Kardiobeat.ai brings new quality in Holter monitoring.
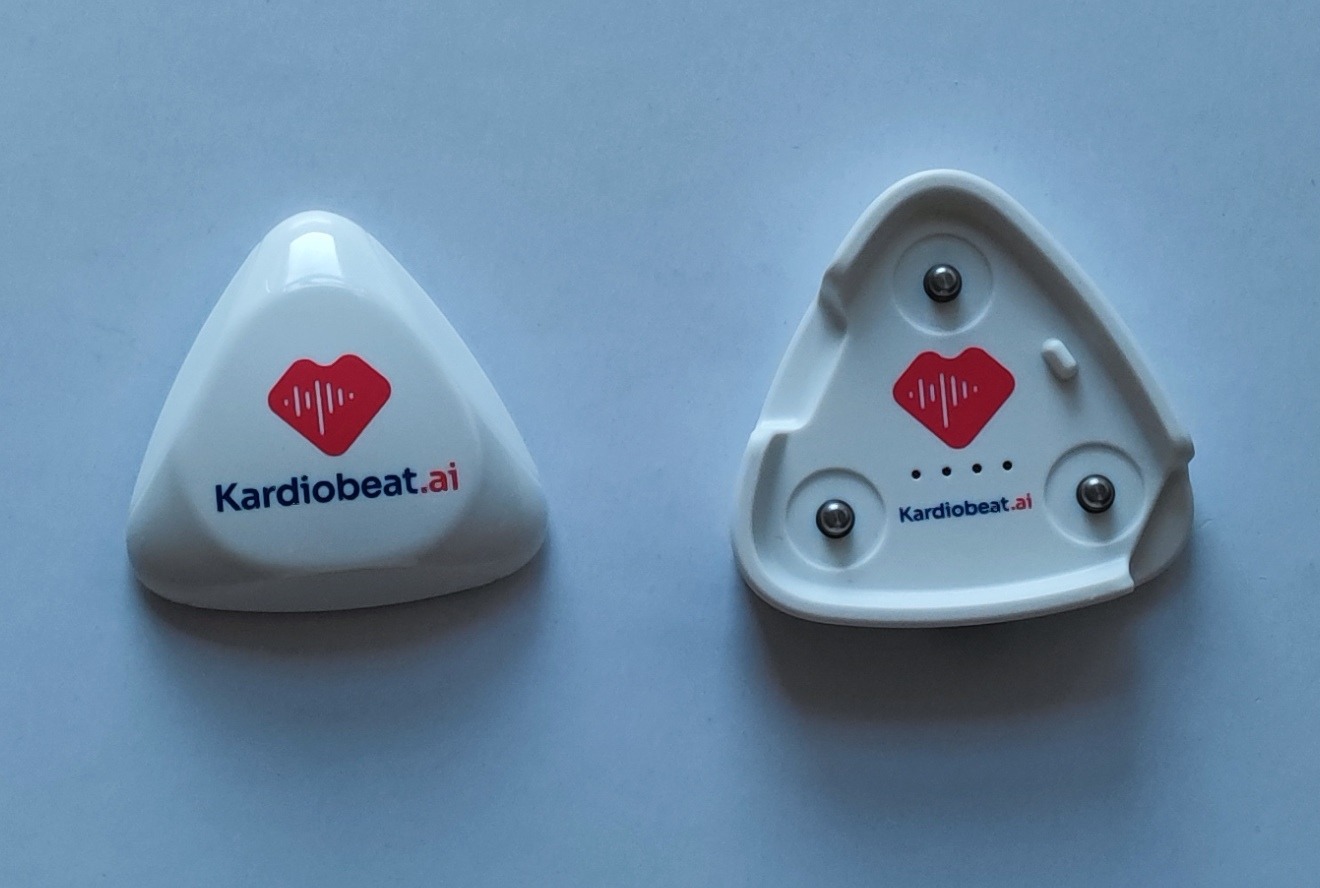

Continuous recording up to 12 days. Powered by AI solution is designed to deliver comprehensive, dynamic reports to make save time to diagnosis.
Real long-time recording with one battery charge
Record up to 12 days
Low signal artefacts
Filter signal processing integrated
Bluetooth capability to view signal via APP
Real long-time recording with one battery charge
Record up to 12 days
Low signal artefacts
Filter signal processing integrated
Bluetooth capability to view signal via APP
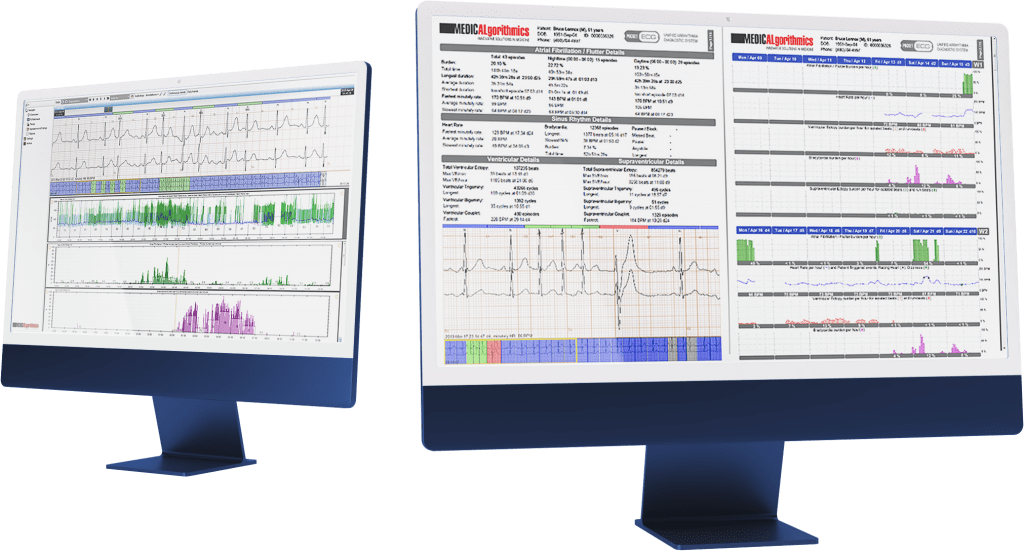
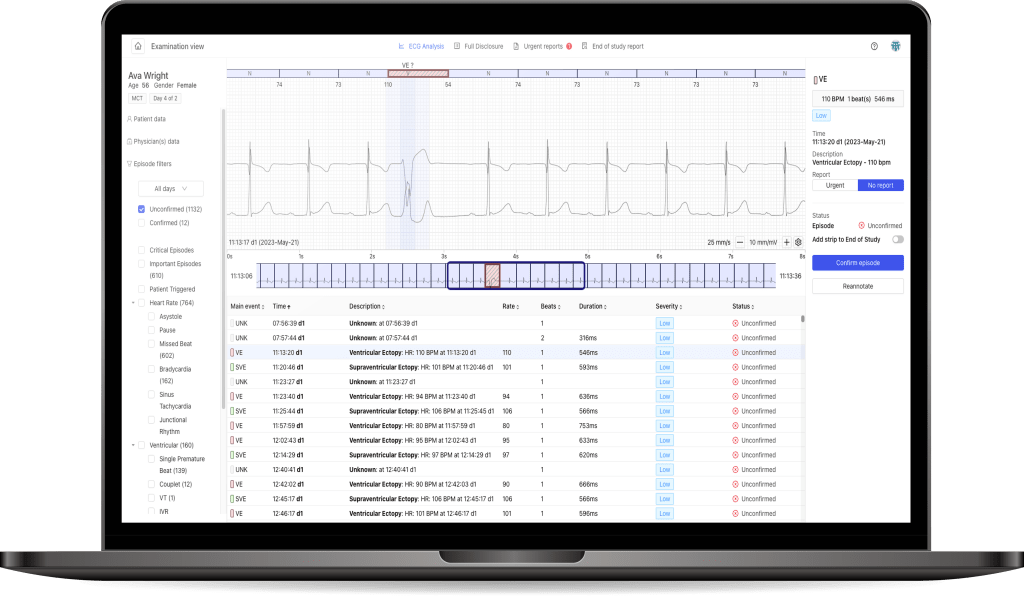
Easy and fast ECG analysis by use PocketECG PCClient software
Check the ECG signal quality in real tume- just use liveECG Viewer

System advantages – patient
High wearing comfort- at home or at hospital
Completely cable- free
High quality of sleep
Low weight, small size& no restriction of movement
Take a shower? IP54 protection class
Discreet: invisible to wear under the clothes
Easy to use and applicable
No expensive and sensitive patient cable (no cable break)
Reusable electronic circuit
Very small effort for cleaning and disinfection
Standard decentral electrodes: 1-3 days wearable
Rechargeable battery

Administratorem danych jest Medicalgorithmics S.A. z siedzibą w Warszawie (02-001) przy Jerozolimskie 81. Dane będą przetwarzane w celu udzielenia odpowiedzi na wysłane zapytanie (podstawa prawna: uzasadniony interes administratora), marketing (podstawa prawna: uzasadniony interes administratora). Pełny tekst klauzuli można znaleźć na stronie Polityki Prywatności.
About us
Our History
Management Team
Partners
Work with us
Job offers
Recommend an employee
Application form