System DRAI (DeepRhythmAI) registration by US Food and Drug Administration (FDA)
Current Report No 51/2022
Date: 28.07.2022.
Legal basis: Article 17.1 MAR – confidential information
The Management Board of Medicalgorithmics S.A. (hereinafter the “Company“) announces that on July 27th, 2022 during the night the Company received notification from US Food and Drug Administration (hereinafter “FDA”) about registration of DRAI system (DeepRhythmAI, hereinafter “DRAI”) in accordance with the Traditional 510(k) procedure.
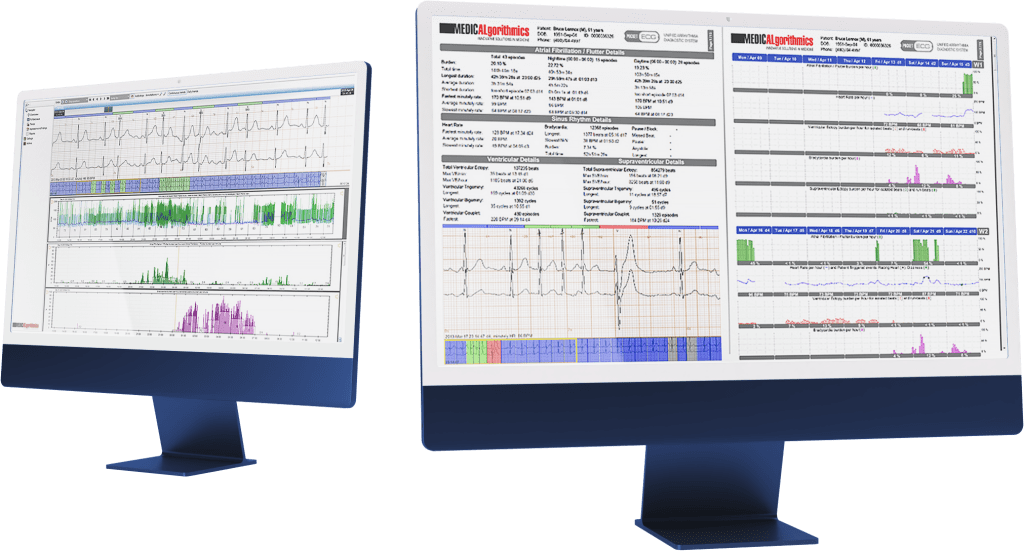
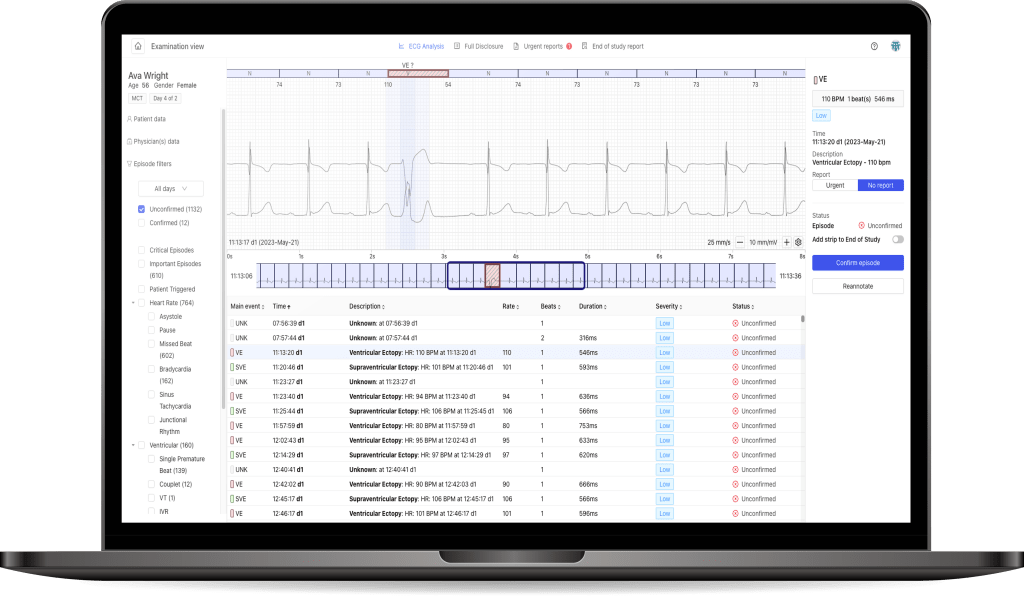
DRAI is a cloud-based artificial intelligence algorithm, that analyses all the heart beats in the processed ECG signal and based on that classifies them as either correct or arrhythmic.
The US market clearance granted by the FDA confirms, that the system features sensitivity and precision of classification on par or above the latest competing technologies. DRAI is able to recognize a broad array of arrhythmias. Thanks to these capabilities DRAI enables faster and more precise diagnosis of the patients. It is our fundamental technology, which will support both current and future products of the Company. DRAI has been designed to work with devices created by the Company, as well as integrating third-party devices, which will open up new business possibilities. These include the capability to offer ECG signal processing services for devices other than PocketECG. Providing this type of service and functionality to external entities, in line with the Management Board’s observations, would correspond to the market needs of the Company’s business partners. The current clearance allows tu use DRAI with devices with similar properties to PocketECG, but the Company is planning to extend the clearance for further device classes.
The Company’s analyzes carried out as at the date of publication of this report show that the implementation of DRAI together with the next generation of the PocketECG system , which is under development, may reduce the workload of ECG signal classification to about 50%, which could bring tangible benefits, including increasing productivity, to business partners who would choose to use this system.
The Company intends to make DRAI available to partners operating on its devices together with the new generation of the PocketECG system not earlier than in the second half of 2023. In parallel, it will analyze the possibility of using DRAI to analyze the ECG signal from other devices.
In the opinion of the Management Board of the Company, obtaining this approval is an extremely important milestone in the Company’s product development. It is also an relevant component of the competitive advantages on the market and value that the Company can present today as a part of its strategic options review and offer to existing and potential business partners.