Subject: Information on Medicalgorithmics S.A. signing an agreement with a NASDAQ-listed tech company that is a leading entity in the non-invasive cardiac monitoring diagnostic sector in the US market
Current Report No.: 30/2023
Date of preparation: 10 October 2023
Legal basis: Art. 17 para. 1 of the MAR Regulation – confidential information.
The Board of Medicalgorithmics S.A., based in Warsaw (“Company”; “Issuer”), announces that on 10 October 2023, the Company entered into an agreement with one of the top 3 leading entities (“Partner”) in the non-invasive cardiac diagnostic and monitoring industry based in the United States, primarily operating in the US market, and publicly listed on NASDAQ.
The purpose of the Agreement is to allow the Partner to evaluate the potential use of the Company’s technology integrated with the Partner’s EKG signal recorder. To this end, the Company’s software will be integrated with the Partner’s EKG signal recorder by developing dedicated software essential for this evaluation (“Software”). As per the Agreement, after this evaluation, the next phase of collaboration may be a separate agreement regarding the joint undertaking of the CE certification process (MDR EU Certificate) and obtaining other required permits for commercializing the Partner’s integrated technology with the Company’s software. In line with the Agreement, if the Company obtains the EU MDR (EU Certificate) only for the dedicated Software within 6 (six) months of application submission, the Company will receive a fee of $250,000.00 USD (approx. 1.1 PLN million). Furthermore, the Agreement confirms both parties’ interest in entering into a partnership agreement concerning the Partner’s continued use of the Company’s products and services.
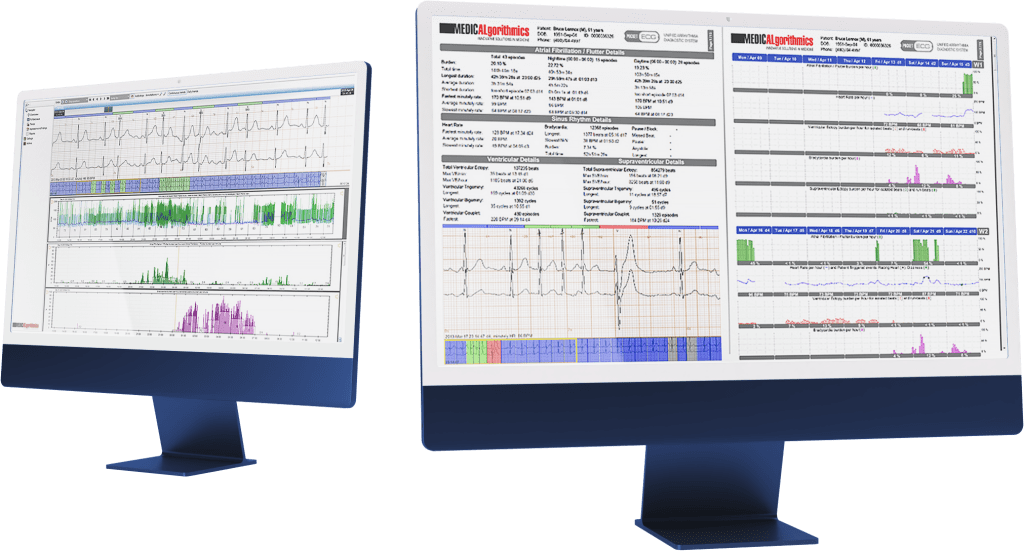
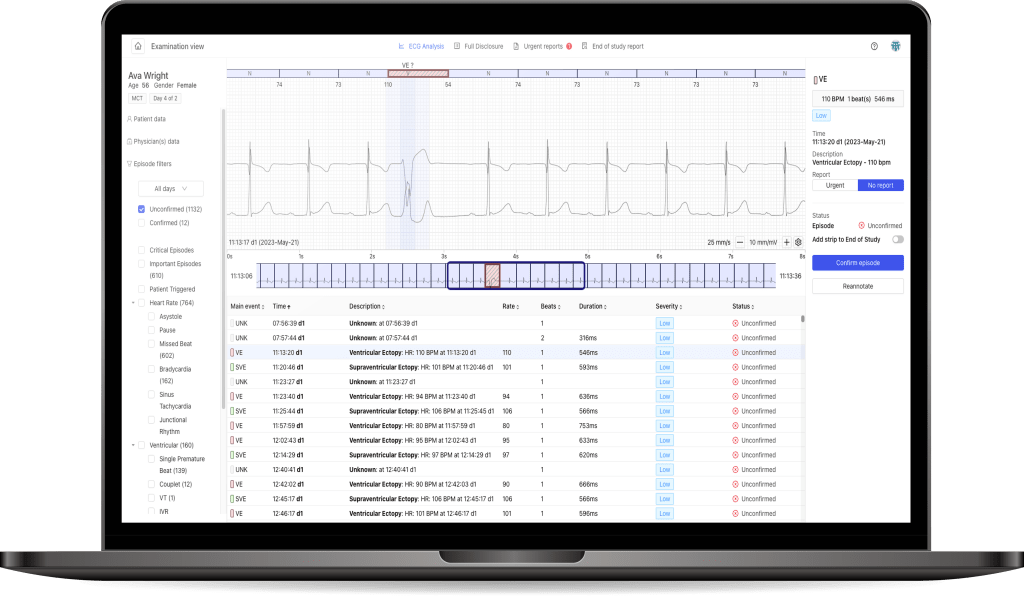
For the Agreement, the Company will utilize its artificial intelligence-based cardiac analysis tools and software and tailor them for processing EKG signals from the Partner’s devices. After completing the software integration of the Company with the Partner’s devices, resulting in the dedicated Software creation, the parties will collaborate for the technical assessment phase. During this cooperation stage, the Agreement foresees the Partner creating teams that will be trained by the Company to handle the Software. The Company will train designated Partner employees on the Software, enabling the Partner’s employees to evaluate workflow, clinical interface tools, system performance, and EKG analysis.
The Agreement aligns with the Company’s strategy adopted after Biofund Capital Management LLC became its major shareholder and published in the form of the Issuer’s current report No. 16/2023 dated 19 June 2023. The Company is now in the phase of implementing this strategy by acquiring commercial partners in a non-exclusive model. The Company has informed of lesser-scale agreements concerning integration services or letters of intent in press releases.
Upon assessing the US market, the Company determined that the counterparty with which the Company signed this Agreement is a significant player in the US market, it is one of the top 3 entities in the non-invasive cardiac diagnostic and monitoring industry in the USA, both in terms of the number of patients they monitor annually and the yearly revenues generated from cardiac ECG monitoring, publicly listed on NASDAQ. The initiation of this collaboration, which foresees the technology evaluation, integration with the counterparty’s system, and dedicated software creation, has significant implications for the Company’s strategy implementation. Concurrently, the Company announces that the parties are actively examining further collaboration possibilities. If a decision is made regarding continued collaboration, a separate agreement on this matter will be entered, which the Company will promptly report in a separate current report.