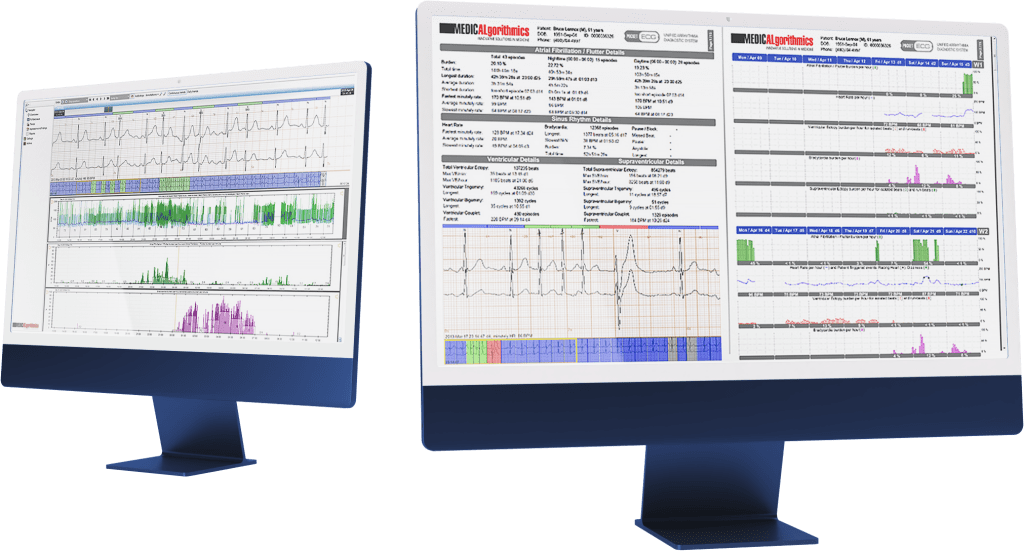
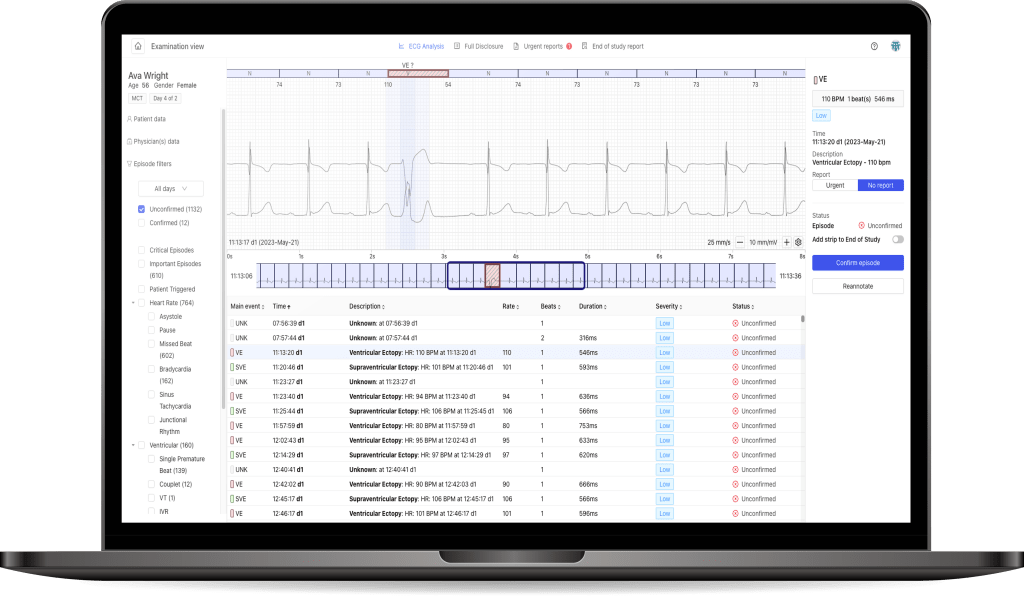
After three quarters of 2017, the Group gained PLN 152.8 million revenue on sales (increase by 64% YOY), and doubled the operational and net profit.
The number of requests for payment from insurers due to the examinations performed with PocketECG reached over 70 thousand this year (increase by 63% YOY).
New sales specialists are recruited in the U.S. sales department and a new remuneration system has been adopted to acquire new customers faster.
A new product – a system for cardiovascular rehabilitation (PocketECG CRS) – met with positive physician and patient opinions after pilot implementation in Poland. Medicalgorithmics S.A., a provider of cardiac diagnostic solutions, has published financial performance results for the third quarter of 2017. The Company has continued to implement its strategy, relying its operations on the innovative PocketECG system. The consolidated Group sales mainly include the revenue on medical services provided in the U.S. and generated by a subordinate company Medi-Lynx Cardiac Monitoring, LLC and revenue on subscriptions generated by Medicalgorithmics S.A. from the cooperation with other strategic partners.
Double-digit increase in revenue and number of requests
After three quarters of 2017, the Group’s revenue was PLN 152.8 million (increase by 64% YOY), and in the third quarter alone the revenue was PLN 50.3 million (increase by 25% YOY). The Groups’ sales derive from the number of diagnostic services provided in a given period by Medi-Lynx customers. After three quarters of 2017, the number of payment requests submitted to insurance companies in relation to provided diagnostic services reached a record 70 thousand, which is 63% more than in the same period last year. In the third quarter, 24 thousand requests were submitted (increase by 65% YOY).
Significant increase in sales and number of performed examinations was possible thanks to the increased scale of Group operations which was influenced by taking over the customers of AMI Monitoring, Inc. and including the financial results of Medi-Lynx in the consolidated results for the entire analyzed period.
The difference between the annual revenue dynamics and the dynamics of the number of requests results from the fact that in 2016 the revenue included subscriptions paid by AMI Monitoring Inc. with which we stopped to cooperate at the beginning of 2017.
Higher business profitability
In the first three quarters of 2017, the Group achieved high profitability levels. The operating profit reached PLN 31.9 million (+188% YOY), EBITDA increased to PLN 41.5 million (+143% YOY), and the net profit attributable to the parent entity shareholders reached PLN 18.2 million (+97% YOY). The operating margin of the Group after three quarters reached 21%, which is 16% more than in the same period last year, and the EBITDA margin increased to 27% in 2017 in comparison to 18% in 2016.
At the end of the third quarter, the Group debts stayed at a low, safe level. The net debt/EBITDA coefficient at the end of September 2017 was at 0.6x, and the general debt index was 0.3x. In the third quarter of this year, the Group cash level increased by PLN 11 million to 30 million at the end of this period.
Development on the global market
The Board maintains the goal to increase the annual Group revenue by at least 20% and achieve EBITDA over 30%. To achieve the planned results, the Group is carrying out several activities to increase sales and maintain control over costs at the same time. On the U.S. market, in the subsidiary Medi-Lynx, in the last quarter we changed the remuneration system in the sales department to stimulate even higher rates of customer acquisition. Besides, new sales representatives are being recruited.
The key to the Group growth lies not only in strengthening its position on the U.S. market but also in the sales increase on other markets and developing new products. “Lately, we have started cooperation with a trading partner from Denmark, who also operates on other Nordic countries. Nowadays, we are carrying out pilot implementation of PocketECG in a large Copenhagen hospital. As many as 59 of our diagnostic devices monitor the hearts of over 500 patients. Very good opinions on our system foretell chances to develop sales in these countries,” Marek Dziubiński, the President of the Board of Medicalgorithmics S.A., said.
The Group also continues to work on new products. The cardiovascular rehabilitation system has been successfully implemented in a large Cracow hospital. Over a hundred patients supervised by several dozen physicians have benefited from remote rehabilitation using PocketECG CRS. “The law changed this year in Poland and hybrid cardiac rehabilitation shall be reimbursed, so many Polish hospitals want to test our products. It is a great opportunity to prepare for entering our target market, i.e. the United States. We submitted an application to FDA to register our cardiac rehabilitation system and we expect to obtain a positive decision by the end of the first quarter of 2018. Next year, we are planning to start pilot implementations on this market,” Marek Dziubiński said.