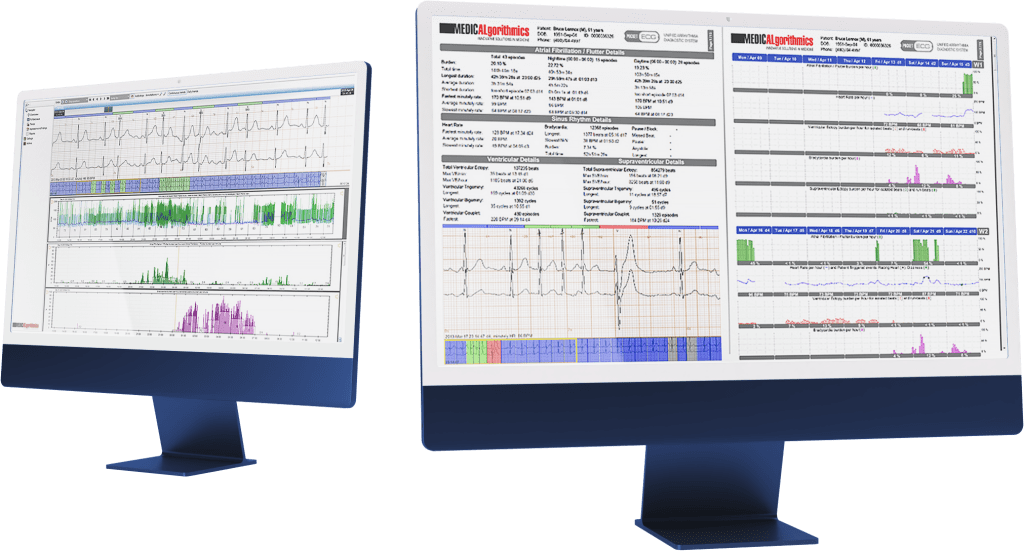
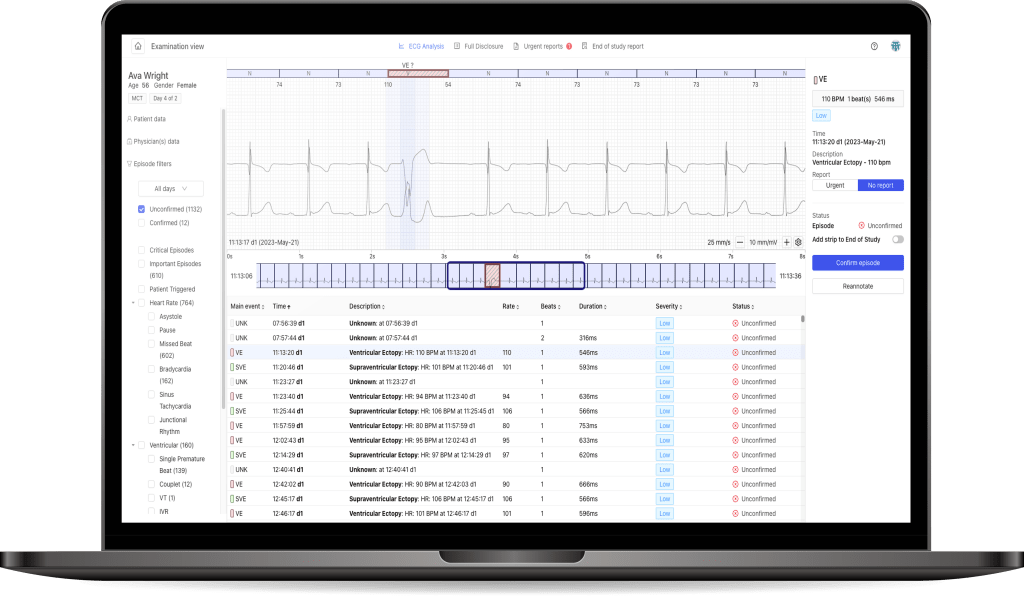
Medicalgorithmics, listed on the Warsaw Stock Exchange, has been selected by AnaCardio, a Swedish clinical-stage biopharmaceutical company, as a cardiac safety monitoring equipment provider in a clinical trial, conducted at leading clinical heart failure centers in the European Union and the United Kingdom. Approximately 80 heart failure patients will participate in the study with the use of the PocketECG 3 device. Medicalgorithmics is also in talks with potential partners on participating in further clinical trials in the US market, among others.AnaCardio, has selected Medicalgorithmics as a cardiac safety monitoring equipment provider (PocketECG 3) in the GOAL-HF1 clinical trial. This is a double randomized and double-blind Ib/IIa dose escalation and cohort expansion clinical trial in patients with heart failure and reduced left ventricular ejection fraction (HFrEF).
AnaCardio recently announced the enrollment of the first patient in the GOAL-HF1 clinical trial. It is expected that the study will be completed at the end of 2024.
“We are thrilled to be part of this prestigious clinical trial, which is being conducted on four major European markets, based on our cutting-edge technology. Our solution plays a vital role in the trial. This is an important project for us, providing evidence of the highest quality of clinical data provided by our PocketECG technology. Participation in this clinical trial opens opportunities for us to reach a new group of potential customers. We are seeing that pharmaceutical companies recognize the immense potential of our solutions,” said Maciej Gamrot, Chief Financial Officer at Medicalgorithmics.
The PocketECG 3 device provides constant cardiac monitoring 24/7, allowing patients participating in the trial to spend time at home, without the need for prolonged monitoring at hospitals.
“Drugs under development may cause various arrhythmic changes among some patients. Importantly, only our PocketECG 3 device can identify them in real time and notify the doctor of a severe cardiac event, providing information for immediate decision making and acting in a timely manner” said Jarosław Jerzakowski, Chief Commercial Officer at Medicalgorithmics. “We have the ambition and potential to create new business in the clinical trial segment, which will provide us with a new revenue stream. We are currently in discussions with potential partners regarding participation in further international clinical trials, including the strategically important American market” – concludes Jarosław Jerzakowski.